Portfolio by intervention
Portfolio by disease
Collaborative clinical trials and clinical studies

38
Sub-Saharan Africa countries hosts recruitment sites of EDCTP-funded collaborative clinical studies.
16% (25)
of the clinical trials involve post-licensing (phase IV) studies with a view to influencing health policies and practice and optimising the delivery of medical interventions for the wide-range of sub-Saharan African health systems and diverse populations.
14% (49)
of all studies target pregnant women and their children. Other key populations are also involved in the studies, such as newborns and infants (91; 26%), children (123; 35%) and adolescents (125; 35%).
371
clinical studies supported by EDCTP2 since 2014. Of these, 61% (228) are interventional (clinical trials) and 39% (143) are non-interventional studies.
65% (101)
of clinical trials are phase II and III trials of drugs and vaccines which aim to deliver key evidence on safety and efficacy, as well as provide data to support product registration.
Medical interventions
New or improved medical interventions against
poverty-related infectious diseases.

In 439 projects awarded to date.
€824.55 M
Total funding



scroll down
EDCTP’s funding of research and capacity development
2014-2023
Towards EDCTP's objectives
Note:
A further €110.47M for 246 grants was awarded to projects on non-intervention-specific topics.
*EDCTP grants only
By intervention
Note:
A further €58.01M for 153 grants was awarded to projects on non-disease-specific topics.
*EDCTP grants only
**EDCTP. grants only
Collaborative clinical trials and clinical studies
2003-2018
EDCTP’s investment
in research & development

In 192 projects awarded by end of 2018
€447.04 M
Total funding



€23.15 M
in co-funding has been secured through two dedicated calls requiring collaboration with development cooperation initiatives, with co-funding from Sida, USAID, Gavi, The Global Fund, UNITAID, AECID and Médecins Sans Frontières.
4
calls have been launched targeting development cooperation initiatives and involving 11 projects with development cooperation partners and co-funders.
68
private sector entities are involved in EDCTP projects. By end of 2018 , these organisations have received €49.42 M in grant value.
External partnerships
Increase interaction with other EU initiatives, including those linked to development assistance.

60%
of the total EDCTP grant value is allocated to sub-Saharan African institutions (€186 million).
15% (359 of 2,371)
of all participation in EDCTP-funded projects involve private-sector institutions. These institutions were awarded €181.2 M by EDCTP in 2023.
€26.84 M
has been leveraged from partners for the launch of joint or coordinated calls for proposals.
16
sub-Saharan African countries are full members of the EDCTP Association. These members have contributed to a total of €1.21 million by end of 2018 through the Participating States’ Initiated Activities (PISIAs) – research activities within the scope of the EDCTP programme that are funded and implemented by one or more member countries.
External partnerships
Increase international cooperation with public
and private partners.
€198.69 M
cash received from the European Participating States to the EDCTP programme.
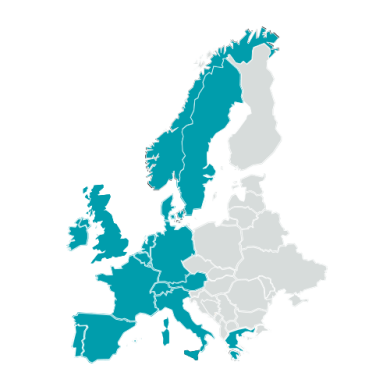
15
European countries are members of the EDCTP Association.
European coordination
Improve coordination alignment and integration of
European National Programmes.
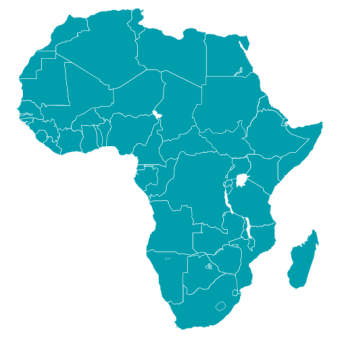
28
sub-Saharan African countries are members of the EDCTP Association by the end of 2023.
215
fellowships grants which supports 362 fellows (145 females and 217 males) to researchers from 39 sub-Saharan African countries.
1,202
trainees from 43 sub-Saharan African countries are supported through EDCTP projects. Trainees include 567 Master's (47%) and 405 PhD (34%).
44
sub-Saharan African countries participate in EDCTP projects involving 310 sub-Saharan African organisations. They receive 60% of total EDCTP grant value.
37
sub-Saharan African countries have received EDCTP support for the establishment of functional regulatory systems and capacities for ethical review of clinical research.
Collaboration and capacity development
Increase cooperation
with sub-Saharan Africa through capacity building for conducting clinical trials according to ethical principles and regulatory standards

38
Sub-Saharan Africa countries hosts recruitment sites of EDCTP-funded collaborative clinical studies.
16% (25)
of the clinical trials involve post-licensing (phase IV) studies with a view to influencing health policies and practice and optimising the delivery of medical interventions for the wide-range of sub-Saharan African health systems and diverse populations.
14% (49)
of all studies target pregnant women and their children. Other key populations are also involved in the studies, such as newborns and infants (91; 26%), children (123; 35%) and adolescents (125; 35%).
371
clinical studies supported by EDCTP2 since 2014. Of these, 61% (228) are interventional (clinical trials) and 39% (143) are non-interventional studies.
65% (101)
of clinical trials are phase II and III trials of drugs and vaccines which aim to deliver key evidence on safety and efficacy, as well as provide data to support product registration.
Medical interventions
New or improved medical interventions against
poverty-related infectious diseases.
Towards
EDCTP2’s
objectives